Latest NEWS
- Aswat Masriya, the last word
- Roundup of Egypt's press headlines on March 15, 2017
- Roundup of Egypt's press headlines on March 14, 2017
- Former Egyptian President Hosni Mubarak to be released: lawyer
- Roundup of Egypt's press headlines on March 13, 2017
- Egypt's capital set to grow by half a million in 2017
- Egypt's wheat reserves to double with start of harvest -supply min
- Roundup of Egypt's press headlines on March 12, 2017
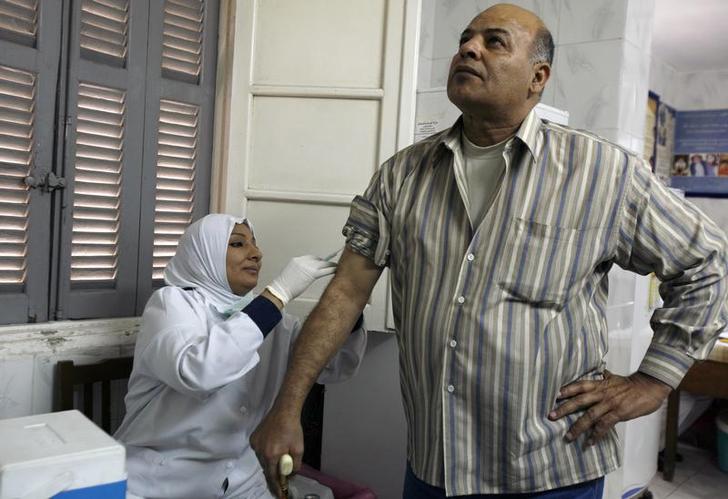
'Clinical Trials': sanctuary for those unable to cover their medical expenses in Egypt (Part 3*)
CAIRO, Jul 20 (Aswat Masriya) - The research center is considered as a regular unit, which applies international standards for clinical trials, and treats the subject of the trial in a separate section isolated from the other patients.
Clinical trials research center, Oncology department at al-Kasr al-Aini
Dr. Noha Abdul Raziq, a pharmacist doctor who works in the Clinical Research Center, Dept. of Oncology, Kasr al-Aini Hospital, said that during a tour in the Center, it was observed that it is divided into separate rooms for preservation of drug samples in the right temperatures according to the type of drug. There was also a room for genetic tests, and another for keeping patient files, records, and informed consents. Some cupboards used for filing patient records were donated by sponsor companies.
There are also air-conditioned rooms dedicated for the administration of doses, with individual chairs for each patient. In the chemotherapy room, there were only two patients. Dr. Abdul Raziq stated, "We try to organize our schedule to avoid overcrowding which is exhausting for the patients. In addition, usually, the number of participants is not so large, not more than 12, due to the high cost of chemotherapy."
Dr. Yasser Abdul Qader, professor of Oncology and Director of the Clinical Research Unit, Dept. of Oncology, Kasr al-Aini Hospital, clarified that, "The research coordinator, for instance, receives around USD 60 per patient throughout the whole period of the trial, which is a very small payment."
Dr. Emad Hamada, Chair of the Oncology Dept., Faculty of Medicine, Cairo University, said, "I receive an annual budget of EGP 4.5 million, while treatment alone costs EGP 13 million. However, the secret lies in charity and donations."
Dr. Heba Khafagi, Lecturer of Oncology in the Dept. of Oncology, al-Kasr al-Aini educational hospital, clarified that cancer patients, unlike other patients, desire to be part of a clinical trial to ensure receiving drugs and treatment for free, due to the high cost of treatment that could amount to EGP 50,000 per month. Moreover, state-funded treatment does not provide full coverage of treatment costs.
She added, "Clinical trials are usually conducted in the third and fourth stages. In the case of rare tumors, trials could be conducted in the second stage."
Clinical trial is Walaa’s only hope
Walaa, a pseudonym, is a patient participating in a lung cancer clinical trial at the Clinical Research Center, Oncology department, al-Kasr al-Aini.
Two years ago, she observed that her left eyelid is sagging. The tests showed that she suffered from lung cancer.
"I learnt of this and I felt scared because I don’t have insurance," Walaa said.
Walaa continued to feel sick. It was discovered that the tumour still exists and is affecting the left side of her body. Only part of the tumour was treated. Another surgery was difficult.
Walaa said, "I was so happy to have an opportunity for treatment. I signed the informed consent form immediately and did not care to read it in detail."
Walaa receives cancer drugs, mainly pain killers, from the Oncology Dept, Kasr al-Aini and from the Cancer Institute. She found this paradoxical. The drugs that are not available are bought from the black market through people working in the Children Cancer Hospital, who steal drugs from warehouses and sell them. She wept saying, "I wish they would know that they are trading in our pain."
Walaa’s experiment ended with no advancement, and her only hope was to start a new clinical trial as her follow-up doctor said. However, she couldn’t get a security clearance to send her samples abroad.

Patient reading document
Clinical Studies Centre, Alexandria University
Established in 2006, this centre is the first in the field and is currently the only comprehensive centre.
Dr. Nihal al-Habashi, Medical Physiology professor, Academic Director of the Clinical Studies Centre, said, "Prior to the establishment of the Centre, the terms 'research coordinator' or 'research pharmacists' were unknown designations."
"We started when Dr. Nadia Zaki, the current Director, and I received a 3-week training in the University of Maryland school of Medicine on Good Clinical Practice (GCP) Guidelines," al-Habashi added.
Dr. al-Habashi said, "We had a successful attempt in the treatment of psoriasis in 2012. We asked the research sponsor company to continue to supply the patients with the drug. However, the Ministry of Health refused, despite the fact that it should be the one stipulating this condition."
Dr. Nadia Zaki, Internist, Faculty of Medicine, and Director of Clinical Research Centre, maintained that the patient would have been treated among other patients. Each physician had a dedicated cupboard where s/he kept files and research drugs dispensed through the Hospital's pharmacy, just like other drugs. "These were trials not sufficiently controlled or supervised." She added, "We did not have any Research Ethics Committee prior to 2005."
Dr. Zaki wonders why so many restrictions are placed on official formalised trials, while some healers are allowed to sell medicinal herbs through their private clinics.
Dr. Nehad Sabri, Medical Coordinator, Clinical Research Centre, University of Alexandria, said, "Since the improvement of trial rates in 2014, a new obstacle was faced, in the name of 'national security'," adding, "this is an important procedure to have all samples from all participating countries analysed in one central lab."
She believed that it was paradoxical that the same study and the same protocol that had been approved before are currently rejected, without giving reasons.
*This is the third and final part of a three-part series investigating clinical trials in Egypt.